Gobierno de la ciudad de Buenos Aires
Hospital Neuropsiquiátrico
"Dr. José Tiburcio Borda"
Laboratorio de Investigaciones Electroneurobiológicas
y
Revista
Electroneurobiología
ISSN: 0328-0446

Electrical synapses between
neurons synchronize gamma oscillations generated during higher level processing
in the nervous system
by
Prof. Michael V. L.
Bennett, D.Phil. (Oxon)
Department of
Neuroscience, Albert Einstein College of Medicine,
1300 Morris Park Ave., Bronx, NY 10461
Contacto
/ correspondence: mbennett [-at-] aecom.yu.edu
Electroneurobiología 2006; 14 (2), pp. 227-250; URL <http://electroneubio.secyt.gov.ar/index2.htm>
Copyright © 2006 by the author. Este trabajo es un artículo de acceso público; su copia exacta y redistribución por cualquier medio están permitidas bajo la condición de conservar esta noticia y la referencia completa a su publicación incluyendo la URL (ver arriba). / This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose, provided this notice is preserved along with the article's full citation and URL (above). Received April 16, 2006; published May 3rd, 2006.
Puede
obtener un archivo .PDF (recomendado)
para leer o imprimir este artículo, desde aquí o de / You can download a .PDF
(recommended) file for reading or printing, either from here or <http://electroneubio.secyt.gov.ar/index2.html

ABSTRACT: Neuron Doctrine no longer encompasses important
aspects of neuronal function. The Neuron Doctrine transformed the 19th-century
view of the nervous system, which saw the brain as a network of interconnected
nerve fibers. A century later, the modern view holds the neuron as a discrete
cell that processes information in more ways than originally envisaged.
Intercellular communication by gap junctions, slow electrical potentials,
action potentials initiated in dendrites, neuromodulatory effects,
extrasynaptic release of neurotransmitters, and information flow between
neurons and glia, all contribute to information processing. A current view of brain cells allows a more proper if
intricate perspective of how neural information is processed in the nervous
system. A preliminary editorial comment ("A Tale in Two Academes") outlines different
paths that led toward it.

Editorial: A Tale in Two Academes
At the hour of depicting higher nervous processs and the psychophysical
nexus, the Anglo-American academe is by no means monolithic. Yet in general its
views have long been consistent with a nervous system conceived as a system of
commands transmitted by relays, as it was put by the celebrious definition by
Louis Lapicque (1932) forwarded in the first volume of the Nouveau Traité de Psychologie edited by George Dumas. In contrast,
the Argentine-German neurobiological tradition, foreign both to the influences
of neuronism and behaviorism, evolved upon the turning-point models forwarded
since 1906 by Christfried Jakob. As it locally is fairly known, these models in
their earliest version presented the higher nervous dynamics as taking place
between "stationary waves" of neuroactivity kept by
"reverberating microcircuits" in the gray, whose unsuitableness to
account for long-term episodal memory – a prime concern for a neurobiology
chiefly carried out in asylums, with a considerable proportion of dementized
insanes – posed stimulating constraints since the beginning. When von Economo
and Koskinas (1925; see in this journal's Index
the articles devoted to their studies on Jakob's work) collated Jakob's and
Cajal's contributions to the scientific description of the nervous gray, they
mainly considered the less integrative, more anatomical contributions of Jakob
and Cajal. The main disparity among these two scientists, however, was that,
while Cajal embraced the application of the "system of commands by relays"'s view for
the entire neurodynamics, Jakob rather kept beyond it a further level of
integrative action to be physiologically as well as physically investigated. By
the time, this further level was absent also from the descriptions of the
integrative actions of the nervous system in the Anglo-American academe, so
Jakob's appraissals of Cajal's contributions ("Santiago Ramón y Cajal: la
significación de su obra científica para la neuropsiquiatría," La Semana Médica 34, 1935; "El
significado de la obra de Ramón y Cajal en la filosofía de lo orgánico," Humanidades 26, 1938) addressed as well
Cajal's views as those of his Anglo-American eponyms. While accepting a short
number of different hierarchical levels for individual cells in the diverse functional
organization of the neural tracts, Jakob in particular rejected chimaeras such
as "psychical neurons" or "cerebral ducts for thought" (Cajal,
el cauce material del pensamiento).
Nevertheless, in Anglo-American academe Jakob was almost exclusively known by
way of Economo and Koskinas' presentation, in spite of his well-known Atlases (quoted in several theses) and
the exception made by some young people such as Donald Hebb and, in Holland,
some Ariens Kappers' disciples (people from our tradition such as Ramón
Carrillo, Manuel Balado and Braulio Moyano had worked for some time in Kappers'
and German laboratories). Soon later, after 1942, when in peripheral nerves it
was demonstrated that parallel axon's activity is reciprocally influenced by
electric field effects, the electric-field interactions of the mentioned
anatomo-physiological units within the vertebrate's neuropilar volume, i.e. among the "stationary waves
entertained by reverberating microcircuits," were thoroughly discussed
here. As Jakob mentioned it in 1949, even quantum effects in them were
fleetingly considered. But it was in the 1960's, after Jakob's demise (1956),
that the research of our neurobiological tradition – by then proceeding in
comparative isolation, due to external and local circunstances – achieved the
phylogenetic reconstruction (see Crocco, "¡Alma e’ reptil! Los contenidos
mentales de los reptiles y su procedencia filética," Electroneurobiología 12: 1-72, 2004) needed to understand the
complex physiological role of the electrical interactions among those units. It
set the scenario to find out the localization of the causal operations involved
in the psychophysical nexus, as required by the clinical observations of
amnesia recoveries. In the Anglo-American academe, meanwhile, Cajal's system of
commands by relays had instead become a solid prefiguration. Hodologies reigned
without collateral effects. A few young researchers, among them the author of
the present article Prof. Michael Bennett, started shaking them since the late
1950's – and their extensive work opened a new, wide scenario. It brings about
the potential for a better reciprocal understanding of the work done in the two
conceptual realms and, in the circumstances, Prof. Bennett's synopsis becomes
of singular practical value. Let his word led us into the current landscape. MS

Electrical synapses between
neurons
synchronize gamma oscillations
generated during higher level
processing
in the nervous system
OUTLINE: The
Neuron Doctrine transformed the 19th-century view of the nervous system, which
saw the brain as a network of interconnected nerve fibers. A century later, the
modern view holds the neuron as a discrete cell that processes information in
more ways than originally envisaged. Intercellular communication by gap
junctions, slow electrical potentials, action potentials initiated in
dendrites, neuromodulatory effects, extrasynaptic release of neurotransmitters,
and information flow between neurons and glia all contribute to information
processing.
Neural information processing
depends on communication between neurons. This communication occurs primarily
at synapses, sites morphologically specialized for intercellular transmission.
This statement avoids defining “specialized”, and the use of “primarily” allows
for transmitter leakage and electric field effects without clear anatomical
specializations. Moreover, glia may have time-varying influences on at least
the slower neuronal oscillations.
Most if not all neurons express
machinery for chemical transmission by secretion of a neurotransmitter from the
presynaptic element that acts on a receptor in the postsynaptic element.
Neurons also have genes to permit electrical transmission, i.e., where an electrical potential generated in one cell affects
a neighboring cell. Early in development most neurons form gap junctions, which
constitute the common kind of electrical synapse, but only a minority do in the
adult. Gap junctions are formed by connexins, a gene family of ~20 members in
mammals. Cloning of Cx36, a (nearly) neuron-specific connexin allowed
demonstration of the wide distribution of electrical synapses, particularly in
sites where oscillations are prominent. Generally, electrical synapses mediate
synchronization, but lateral spread and forward transmission of excitation also
occur.
Although electrical transmission
can be more rapid than chemical transmission, its speed of action is not necessary
in generating gamma and related rhythms. (Electrical transmission may be
required for the speed of high frequency “ripples”, but the cellular basis of
these externally recorded responses is as yet unclear.) Synchronization at low
frequencies could be driven by chemical synapses, mutually excitatory or, less
obviously, inhibitory. In the latter case, computer simulations show that reciprocal
inhibition superimposed on tonic excitation can result in synchronous
oscillation. Oscillation is driven by the tonic excitation (or can be an
intrinsic membrane property). If one cell doesn’t reach threshold when the
others do, it is inhibited and then is ready to fire with the other cells in
the next cycle.
Most cortical neurons must
“choose” to release either the excitatory transmitter, glutamate, or the
inhibitory transmitter, GABA. Gap junctions permit GABAergic cells also to be
excitatory and to synchronize with other GABAergic cells more precisely than
with inhibition alone. Inhibitory interneurons provide the pacemaker of the
oscillations; principle cells and other downstream elements are synchronized,
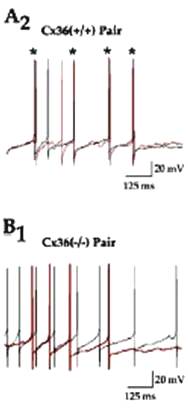
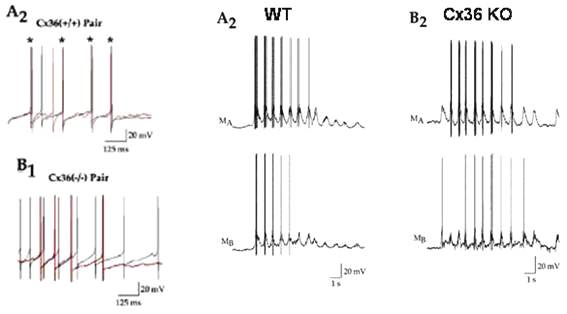
not by excitation but by inhibition. In the Cx36 knockout mouse, inhibitory
interneurons are not coupled and oscillations in the external field are smaller
but not absent. The continued oscillations depend in part on intrinsic membrane
properties or tonic excitation, and reciprocal inhibition mediates some
synchronization. The phenotype is benign, although results of cognitive testing
have not been reported.
New methods of imaging and
recording in vitro and in vivo make feasible characterization of microcircuitry
of modules observed with earlier less inclusive methods. Neurons can express
gap junction forming proteins, and electrical transmission is likely to be
found wherever it is “useful.” The relatively benign behavioral phenotype of
the Cx36 knockout mouse indicates that synchronization of neurons by gap
junctions confers a modest survival advantage in the laboratory, but quite
likely a highly significant one in the real world.
1. Neuron Doctrine no longer
encompasses important aspects of neuronal function
After a century, neuroscientists are rethinking the
Neuron Doctrine, the fundamental principle of neuroscience. A modern view of
brain cells allows a more proper if intricate perspective of how information is
processed in the nervous system.
The formulator of the Neuron Doctrine was primarily
the great Spanish anatomist and Nobel laureate Santiago Ramón y Cajal, arguably
the Watson and Crick of neurobiology and author of Recuerdos de mi Vida (Recollections
of my Life), which every student should read. Cajal argued that neurons
interact at points of contiguity, later called synapses; he and others showed
that there are no points of continuity as proposed by Golgi, one of the
protagonists of the reticular theory. Neurons, Cajal stated, arise through
differentiation of a neuroblast cell to become dynamically polarized: inputs
are to dendrites, outputs are through axons.
Separateness of neurons was an anatomical observation,
at the time as much painstaking to generalize as rewarding. "Dynamic
polarization" was ascertained on the basis of sensory inputs in visual,
olfactory and cutaneous inputs and motor outputs, which data suggested a
similar information flow in the neocortex.
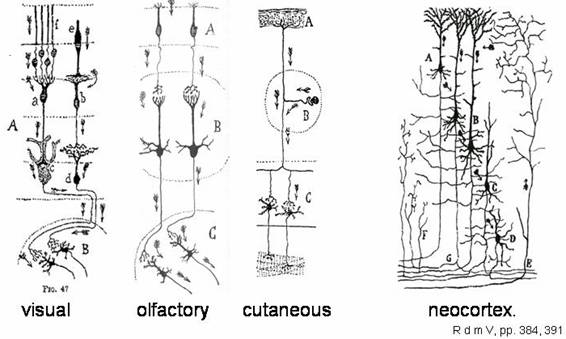
One hundred years since its inception, an examination
of the Neuron Doctrine indicates that it no longer encompasses important
aspects of neuronal function. Technology and research have extended our knowledge
far beyond the simple description that a neuron is an anatomically and
functionally distinct cellular unit that arises through differentiation of a
precursor neuroblast. And neurons are not the single functional units in the sense
envisioned by early proponents of the Neuron Doctrine: non-neuronal
constituents of the nervous system show a variety of unexpected participations
in brain dynamics. If we are to understand complex, higher level neuronal
processes, such as brain function, we need to explore beyond the limits of the
Neuron Doctrine. We can no longer think the nervous system to function as a web
of interconnected nerve fibers.
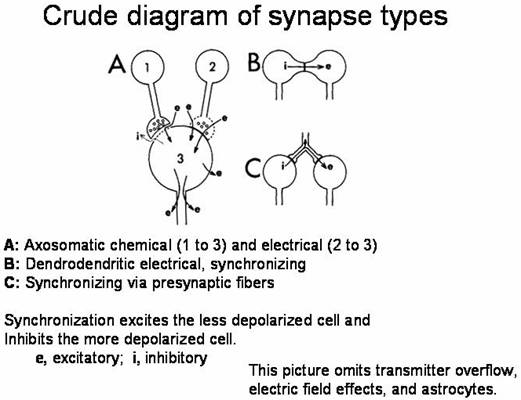
As physiological studies established that conduction
of electrical activity along the neuronal axon involved brief, all-or-nothing,
propagated changes in membrane potential called action potentials, it became
often assumed that neuronal activity was correspondingly all-or nothing, action
potentials spreading over all parts of a neuron. The neuron was regarded as a
single functional unit: It either was active and “firing” or was not. This
dogma began to erode with the advent of microelectrodes that could be inserted
into neurons to record electrical signals.
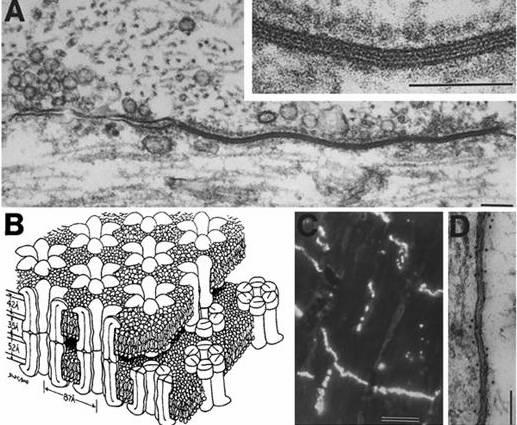
Gap junctions, the most common form
of electrical synapse
Before 1959, it was realized that much of the
information processing by neurons involves electrical events that are graded in
amplitude and decay over distance, developed in the past 50 years – notably
single channel recording, live cell imaging, and molecular biology. Cajal
wisely considered that “neuronal discontinuity… could sustain some exceptions”
to the Doctrine’s definition ("la
discontinuidad neuronal … pudiera padecer excepciones"), as he wrote
in ¿Neuronismo
o reticularismo?'s Conclusión.
Cajal also remarked that "It is clear that future
techniques may contribute new and unsuspected arguments favoring the
reticularist thesis, or other conceptions. A tiny improvement in a procedure's
yield, or a histological discovery of general reaching, may force us to modify
our conclusions. Nowadays, however, such revision does not appear either in
close proximity or even as probable. We can therefore still
adopt, without reservations, the brilliant doctrine of His, Forel, and
Kölliker" ("Claro es que la técnica del porvenir puede aportar
argumentos nuevos e insospechados en favor de la tesis reticularista o de otras
concepciones. Una pequeña mejora en el rendimiento de un método, o un descubrimiento
histológico de alcance general, pueden obligarnos a modificar nuestras conclusiones.
Mas hoy por hoy esta revisión no parece próxima ni probable. Podemos, pues,
adoptar aún, sin reservas, la genial doctrina de His, Forel y Kölliker …"; Cajal, in ¿Neuronismo
o reticularismo?'s opening).
Even so, he could not have foreseen the presence and
role of neuronal gap junctions as one of these exceptions. Furthermore, gap junctions
have been described between neurons and non-neuronal cells such as astrocytes,
a somewhat controversial finding not either conceived in the original Neuron
Doctrine.
These assemblages of protein pores (B, above) form
small aqueous channels of limited selectivity that connect neurons, providing
cytoplasmic continuity. Their collections appear as regions resembling the active
zones of chemical synapses, although there is no chemically mediated signal
transmission and response. We now know that although only a minority of neurons
form them in the adult, gap junctions are widespread in the mammalian nervous
system and function to synchronize neuronal firing.
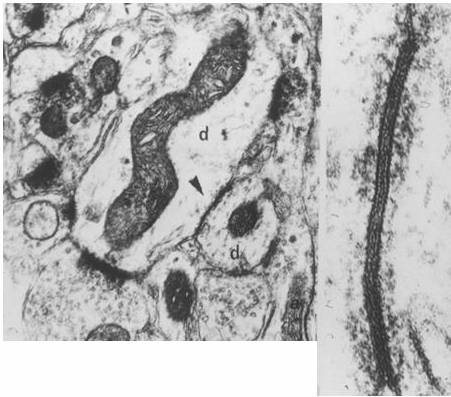
Dendrodendritic gap junctions in primate neocortex
(J. J.Sloper)
They constitute electrical synapses that couple groups
of cells into functional syncytia—in this sense, the reticular concept,
reinvoked.
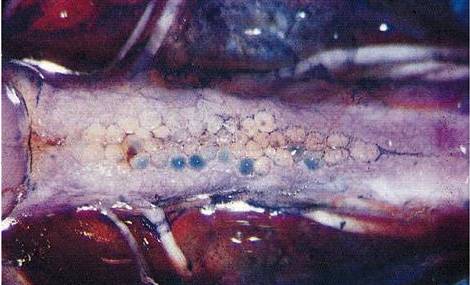
Supramedullary neurons of the
puffer, Spheroides maculatus. (Freud studied similar neurons in the
lamprey: Freud, S., "Über den Ursprung der hinteren
Nervenwurzeln im Rückenmark von Ammocoetes (Petromyzon
Planeri)," Sitzungsbericht der kaiserlichen
Akademie der Wissenschaften LXXV, III Abtheilung; eds. Karl Gerold's Sohn,
Vienna, Jänner bis Mai 1877).
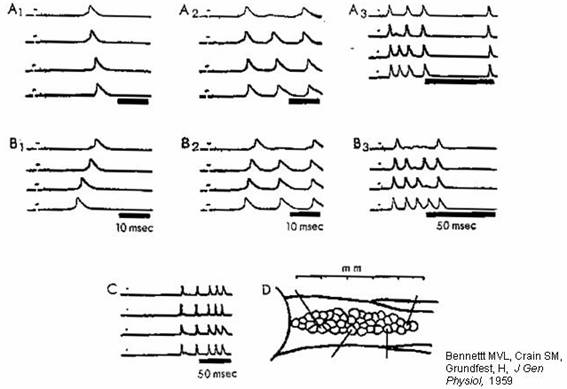
Synchronization of neuronal firing
by electrical coupling. D shows the placement of electrodes in
the fish anatomy (previous figure). Synchronization depends on electrical
coupling: see (next graph) responses to stimulating electrode.
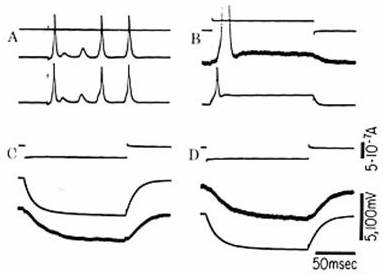
Although gap junctions can behave as
simple electrical resistances between connected cells, an electrical impulse in
one cell by no means inevitably propagates to the other cells with which it
shares gap junctions. In fact, a channel within a gap junction is not
necessarily open, and an entire gap junction may not transmit electrical
current until it is appropriately modified in response to transmission from
chemical synapses of the same, “presynaptic” neuron. This modulation of
channels provides electrical synapses at gap junctions with the plasticity long
considered an exclusive province of chemical synapses at axon-dendrite
junctions (6).
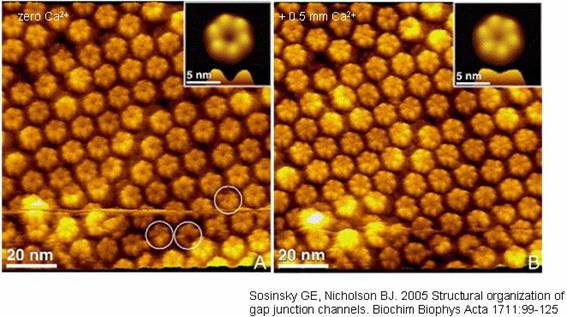
Atomic force microscopy of a split open junction shows
hexameric composition. Elevated Ca2+ causes the apparent pore in the
center of the hemichannel to close.
2. Some recent experimental results
A plethora of neuromodulatory substances, such as
amines and neuropeptides, can reconfigure neuronal circuits into different
patterns of functional connection, capable of a variety of activity patterns
(8). Such neuromodulation remodels neuron behavior and circuitry within minutes
and hours rather than on the millisecond time scale typical of electrical
impulse transmission. In addition, neuromodulatory substances can act at
multiple sites on the neuron, including the axon. For example, some crab (9)
and lobster (10) axons have receptors to amines such as dopamine, serotonin,
and octopamine. When these amines are applied to the axons, these areas can
spontaneously initiate action potentials in a nonclassical mode of integration.
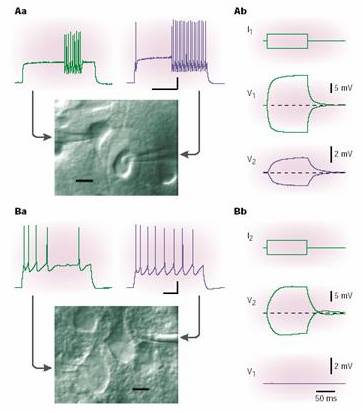
Electrical coupling between a pair of fast-spiking (FS)
GABA interneurons (above) and absence of coupling between pyramidal cells.
Recording pipettes are obvious. When either cell is depolarized or
hyperpolarized, attenuated and slowed potentials are recorded from the other
cell. Demonstration by Hestrin and Galarreta, Nature Rev. Neuroscience 2, 524-433, 2001.
Research during the past ten years has shown that in
many neurons, action potentials can travel backward from the axon and soma
regions into the dendrites. Moreover, under certain conditions action potentials
can be initiated in dendrites, remaining local or sometimes propagating into
the soma to initiate single or multiple spikes of activity in the axon. The
functional complexity of dendrites and the roles they play in synaptic
integration and plasticity are well beyond what could have been deduced from
Cajal’s anatomy or from later somatic recordings. Finally, the function, origin, and diversity
of non-neuronal cells eluded Cajal, because a staining method, which revealed
neuronal structure with brilliant clarity, left major classes of non-neuronal
cells invisible (including microglia and oligodendrocytes). We now know that
some of these non-neuronal cells partake in neuroactivity, through a complex
superposed system, mentioned below, whose time scales are slower than those of
neuronal exchanges.
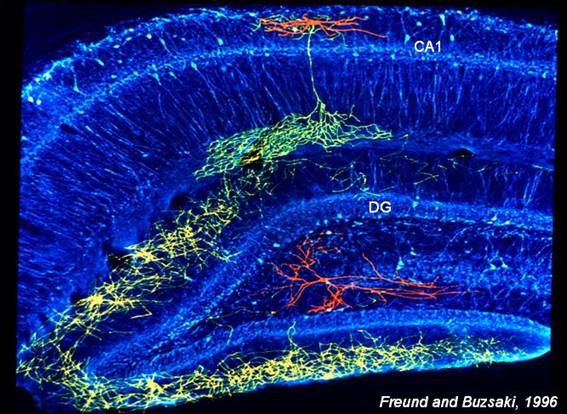
Hippocampal inhibitory axons (yellow
and green) can synapse on many neurons. Cell body and dendrites shown in red.
Dendrites contain a mosaic of voltage-gated ion
channels (13). The types, densities, and properties of these channels are very
diverse among classes of neurons (and even within a single class), and these
channels regulate, on wide-ranging time scales, how a neuron responds to the
thousands of incoming synaptic events that impinge on its dendrites.
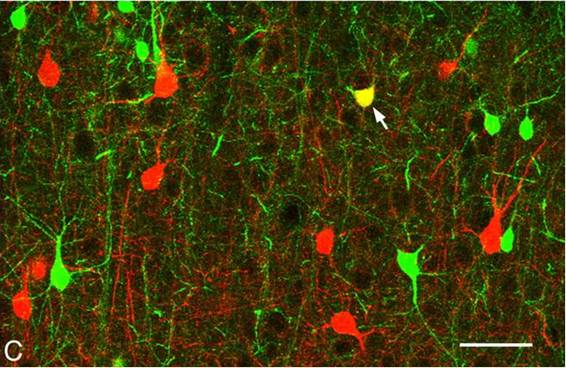
While parvalbumin (PV) is red,
showing fast-spiking (FS) neurons on the above image; calbindin (CB) is green,
showing low-threshold-spiking (LTS) cells. Neocortical inhibitory (GABAergic)
interneurons are mostly either PV- or CB- positive. Source: Fukuda, T., Kosaka,
T., Singer, W., Gauske, R. A., "Gap junctions among dendrites of cortical
GABAergic neurons establish a dense and widepread intercolumnar network," J. Neuroscience 26, 3434-3443, 2006.
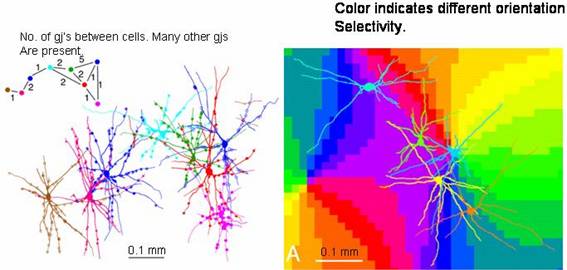
In the figure below, those tiny Cx36 immunoreactive
spots (red) between PV neurons are actually gap junctions:
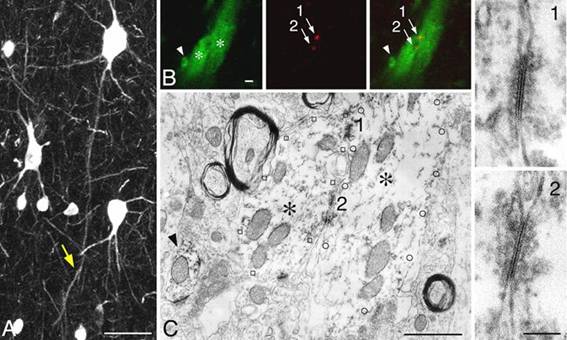
The dendrites of individual inhibitory
neurons extend across the borders of orientation columns, as shown in 2006 by
Fukuda, Kosaka, Singer, and Gauske ("Gap junctions among dendrites of
cortical GABAergic neurons establish a dense and widepread intercolumnar
network," J. Neuroscience 26,
3434-3443). It demonstrates that in visual cortex inhibitory dendrites extend
long distances to make gap junctions with other inhibitory neurons. Thereto,
these dendrites cross borders between functional domains.
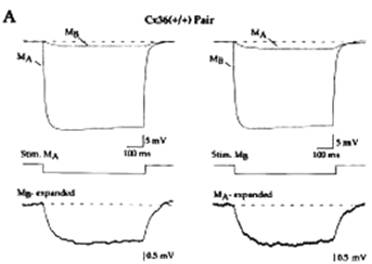
Now, we should consider that the charging time of the
postsynaptic capacity introduces delay at electrical synapses; another way of
describing this feature is saying that they act as low-pass filters. Then there
is further delay in reaching threshold and propagating the action potential to
the next cell. (One may smile mulling over the fact that Helmholtz was right,
nerve conduction velocity being surely finite even here.)
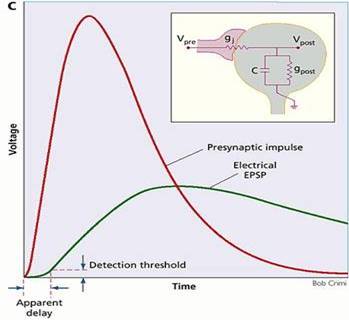
Gap
junctions in conjunction with postsynaptic capacitance behave as low pass
filters. In the box, the equivalent circuit is given. The junctional
conductance, gj, connects the presynaptic cell to the postsynaptic
conductance, gpost, and capacitance, C, in parallel. The curves
show calculated presynaptic impulse and postsynaptic potential for a reasonable
ratio of impulse rise time to coupling time constant, but with a DC coupling
coefficient of unity. The postsynaptic potential is attenuated and slowed. The
slowing introduces a measured synaptic delay.
The transmitted spikes can led to net inhibition,
depending on the afterpotential, as shown in the following results
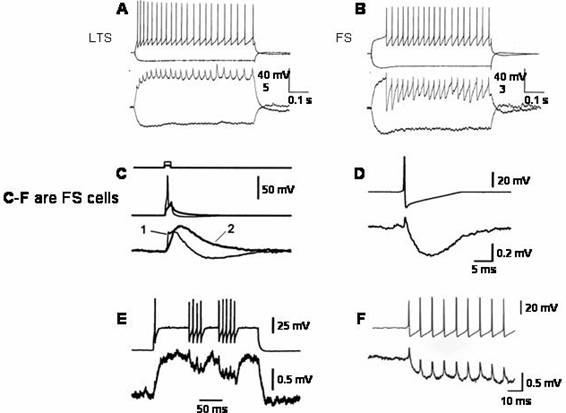
Low pass
filtering at interneuronal electrical synapses: hyperpolarizing afterpotentials
may lead to inhibition (A) Electrical PSPs of coupled LTS (low threshold
spiking) interneurons summate so that
depolarization increases during a burst of
impulses. (B) Electrical PSPs of
coupled FS (fast spiking) interneurons
have an initial depolarizing spikelet,
but the afterhyperpolarization then decreases
the depolarization to below the level
at which presynaptic firing began. (A) and
(B) from Deans,
M.R. et al., Neuron 31, 477-485, 2001. (C) Electrical PSPs from brief
depolarizations just suprathreshold
and subthreshold for an impulse in
one of a coupled pair of FS interneurons.
The subthreshold presynaptic depolarization
(2) causes a monophasic and slowed postsynaptic
depolarization. The suprathreshold stimulus
(1) causes a biphasic postsynaptic
potential due to transmission of the
afterhyperpolarization; the depolarizing phase
decays more rapidly in (1) than in (2). (D)
Averaged postsynaptic responses of single
presynaptic impulses evoked by a steady
depolarization. These impulses have a greatly increased afterhyperpolarization measured from the depolarized potential just prior to the impulses compared to those initiated by a brief stimulus. The resulting PSP has a large negative going, inhibitory component. (E) Postsynaptic responses in a coupled
pair of FS neurons. When the stimulated cell generates a burst of impulses, the
postsynaptic response has an initial
depolarizing spikelet followed by relative hyperpolarization with smaller
superimposed spikelets. (C)–(E) from M. Galarreta and S. Hestrin, PNAS 99, 12438-12443, 2002. (F)
An expanded sweep of a similar burst to those in (E) showing the spikelets
superimposed on the hyperpolarization. (F) by the same, Nature Rev. Neurosci. 2, 524-433, 2001.
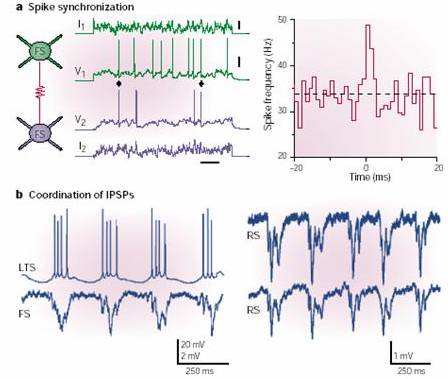
As also shown by M. Galarreta and S. Hestrin in Nature Rev. Neurosci. 2, 524-433, 2001
(next image), the coupling of interneurons synchronizes IPSPs in follower
cells, thereby establishing an activity that is electroencephalographically
recorded as gamma rhythm. LTS (low-threshold spiking) cells inhibit FS
(fast-spiking) cells and RS (regular spiking) pyramidal cells.
Cross-correlogram is in the upper-right quadrant:
Cx36 synchronizes ocillations in the
gamma band with little effect on frequency, as shown by S. G. Hormuzdi and H.
Monyer in Neuron 31, 487-495, 2001:
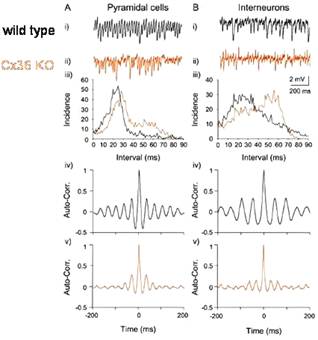
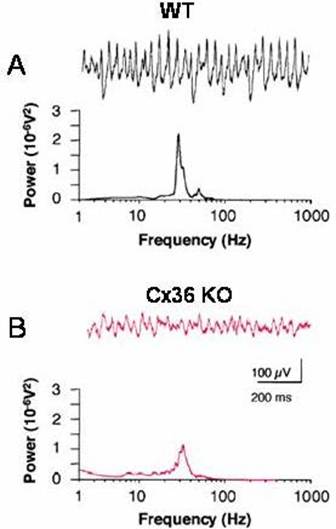
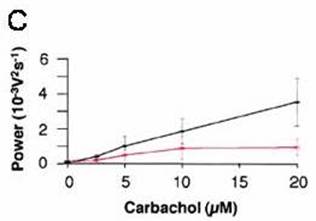
On brain slices of the Cx36 KO mouse, with the gamma
induced by carbachol, bath-applied drug and extracellular recording, Hormuzdi
and Monyer (loc. cit.) observed that
the gamma power is reduced, but the frequency is the same:
As observed by J. M. Christie et al. (Neuron 46,
761-772, 2005), olfactory bulb mitral cells with dendrites in the same
glomerulus are coupled and tend to fire syncronously:
No
synchronization in the Cx36 KO mouse.
Yet, as communicated by the same source, low frequency
oscillations of mitral cells do not require Cx36 for synchronization of bursts
if Glu uptake is blocked:
How do we know that the observed coupling is mediated
by gap junctions? For voltages small enough that voltage gating of gap
junctions is negligible, coupling via (nonrectifying) gap junctions has the
same electrical characteristics as that mediated by a small region of cytoplasmic
continuity, i.e., the junction acts
like a decrease in conductor diameter that decreases longitudinal conductance.
Morphological data can demonstrate gap junctions
between classes of cells that are coupled physiologically, but marking a pair
of coupled cells and then demonstrating gap junctions between them by electron
microscopy is difficult. More indirectly, gap junction mediation of coupling
between interneurons is indicated by sensitivity to blocking agents, which
include heptanol, octanol, halothane, carbenoxolone, α-glycyrrhetinic
acid, anandamide, oleamide, and fenamates. Cytoplasmic continuity, where
tested, is unaffected by these agents.
Another indication of coupling via cytoplasmic
continuity, rather than via gap junctions, is cell-cell passage of larger
molecules that do not cross gap junctions, such as fluoresceinated dextrans;
injection of a combination of gap junction- permeant and -impermeant molecules
into cells in principle enables one to distinguish between the two mech- anisms.
The possibility that inhibitory interneurons are coupled by cytoplasmic
continuity seems excluded by the (near) absence of biocytin or Neurobiotin
coupling.
3. Connexins and pannexins
A bird-eye view on connexins may be in order here.
Connexins, the proteins forming gap junctions, are encoded by a gene family
with at least 20 members in mammals. They are commonly named by their predicted
molecular mass to the nearest kDa, with a prefix for species where necessary. A
later connexin with a kDa number already occupied gets an additional
significant figure, as in Cx30.1. Given that the human genome and much of the
mouse genome have been sequenced, few additional mammalian connexins are likely
to be found. Connexins (as members of a gene family) have conserved sequences
and exhibit a common membrane topology. Different connexins assemble to form
junctions that differ in single channel conductance, gating, permeability depending
on both size and charge, and temporal and spatial patterns of expression. At a
gap junction, each cell provides hemichannels or connexons that dock one to one
with hemichannels in the other cell. Hemichannels are hexamers, homomeric if
they are comprised of one kind of connexin and heteromeric if they are
comprised of more than one kind. Co-expression of multiple connexins is common
in cells, and heteromeric hemichannels do occur, although their prevalence and
stoichiometry are poorly known.
At least ten connexins are expressed in the mammalian
central nervous system but with differing cell specificity. Cx36 is the
principal neuronal connexin in the adult. Cx45 is strongly expressed in the
brain at the mRNA level for the first two weeks of development and is largely absent
in the adult except for hippocampal CA3, thalamus, and cerebellar granule
cells. Studies by freeze fracture replica immunolabeling (FRIL), which are
precise but at this time still limited in number of cell types examined,
indicate that the studied neurons do not express Cx30, Cx32, or Cx43. Other
methods of mRNA and protein detection provide evidence for expression of Cx43
and/or Cx45 by olfactory neurons, mitral cells of the olfactory bulb, locus
coeruleus neurons, and motoneurons. Horizontal cells, which are extensively
coupled probably in all vertebrates, do not express Cx26 or Cx36 and may be
coupled by Cx57. High-frequency discharges in hippocampus, which appear to be
mediated by electrical synapses, persist in the Cx36 knockout animal; these
data support the existence of one or more additional connexins expressed by
neurons.
An intriguing possibility is the existence of yet
another class of proteins that form gap junctions in mammals. In protostomes,
such as C. elegans and Drosophila, gap junctions are composed
of members of a gene family that is unrelated to connexins but exhibits a
surprising degree of evolutionary convergence. The absence of connexins in protostomes
can be asserted with some confidence now that the genomes of Drosophila and C. elegans have been sequenced. To those of us who had come to
regard connexins as our gap junction family, it was a bit of a shock when data
mining in the human genome disclosed three homologs of the worm and fly gap
junction genes, a family evidently unrelated to the connexins.
These genes were originally termed innexins for
invertebrate gap junction forming proteins, an inappropriate name at the time
(1998) given that ascidians, which are invertebrates, had gap junctions with
Cx32-like immunoreactivity. Y. Panchin et
al. ("A ubiquitous family of putative gap junction molecules,"
Curr. Biol. 10, R473–R474, 2000) proposef the name “pannexin” for universal
(pan) nexus (connection) protein for both the mammalian and invertebrate
proteins in this family, a proposal resisted by others, who retain innexin for
the protostome line of bilaterally symmetrically animals (nematodes, mollusks,
annelids, arthropods) and (ignoring the etymology) use pannexin for the
vertebrate homologs. Two of the rat homologs are expressed in the CNS and form
gap junctions when expressed in Xenopus
oocytes. Functionality in the CNS remains to be determined.
Although differences in the sequence indicate that
connexin-based and pannexin-based gap junctions are separate evolutionary
adaptations, there is a remarkable degree of functional convergence including
permeability to molecules of ~1 kDa and block by many of the same pharmacological
agents, by low cytoplasmic pH, and by high cytoplasmic Ca2+. Dual
mechanisms of gating by transjunctional volt- age are found in both classes. In
pannexin-based junctions, the channel diameter is a little bigger, the gap is a
little wider, and the number of channels per unit area is a little lower.
Although the role of pannexins in mammalian tissues is still unknown,
conservation of function has been established for many proteins with real
homologs (not analogs) in both insects and mammals.
What is the survival value of multiple connexins?
There are the obvious functional differences in permeability, gating, and
posttranscriptional regulation of formation and degradation. Formation of
heterotypic gap junctions can be prevented through expression of incompatible
connexins, although many cells expressing compatible connexins do not form
junctions. Differences in transcriptional control may be more important than
the functional differences in the connexins themselves.
4. A recently-achieved panorama
It is indeed ironic that the fundamental tenet of the
Neuron Doctrine – polarized
communication between neurons by action potentials – is heavily influenced by non-neuronal
cells. Namely, by the constituents of the nervous system that form the myelin
sheath around axons and organize ion channels into periodic clusters along the
axon, features that facilitate action potential propagation. We do not yet know
how the spatial distributions of individual ion channels in the surface
membrane of dendrites are established, how this variable localization changes
in response to incoming synaptic inputs and output firing patterns, and how the
channels dynamically regulate excitability during different behavioral states.
Yet we do know that non-neuronal cells act upon them. Besides, they work in a
myriad other ways. Myelinating glia do not fire action potentials, but they can
detect impulses in axons through membrane receptors that bind signaling
molecules. These include ATP and adenosine that are released along the axon,
and also potassium that is released during intense neural activity.
This axon-glial communication violates the Neuron
Doctrine in two ways. Information is communicated between cells at sites far
removed from chemical synapses, and it propagates in a transduced form through
cells that are not neurons. In response to neural firing, glia communicate with
other glia by chemical signaling and gap junctions rather than by electrical
impulses. Chemical synapses have been detected between neurons and a class of
glia (oligodendrocyte precursor cells), undermining a defining feature of
neurons. However, the functional importance of this neuron-glia interaction is
as yet unknown.
Other interesting facts whose import has not yet been
fully elucidated are the following:
1. We now know that during vertebrate embryonic
development, glia can give birth to neurons, challenging Cajal’s conclusion
that neurons develop only from neuroblasts.
2. Astrocytes are now known to communicate among
themselves by means of glial transmitters and neuromodulators as well as by gap
junctions.
3. Moreover, astrocytes can detect neurotransmitters
that are released from neuronal chemical synapses. These transmitters are delivered
via synaptic vesicles into the synaptic cleft and diffuse to perisynaptic
astrocytes.
4. Additionally, neurotransmitters can be released
outside the synapse and detected by perisynaptic glia.
5. In response, astrocytes can regulate communication
between neurons by modifying synaptic transmission through the release of neurotransmitters
and neuromodulators.
As these five facts intimate, there may be a
"parallel" or rather overlapping system of information processing
that interacts with neuronal communication but propagates over much slower time
scales through a functionally reticular network of non-neuronal cells. This
functional reticulum results from gap junction coupling and the omnidirectional
communication that is mediated by chemical messengers released from astrocytes
over much slower time scales.
5. What would Cajal have said?
As cloning of neuron-specific connexins, increased
capability of visualizing cells within brain tissue, labeling of cell types by
transgenic methods, and generation of connexin knockouts have spurred a rapid increase
in our knowledge of the role of gap junctions in neural activity, many new
questions arose. Yet one should not lose view of the most basic, fundamental
ones. So, why electrical synapses, then?
Factors such as speed of action and shorter conduction
delays are important in some escape systems where time is of the essence, what
is only true in small animals. Speed of action and shorter conduction delays
also are important in precise
synchronization. This is not obviously true of gamma waves. Such
features are potentially important in increasing phase velocity – but action
only over short distances. Whereas electrical synapses are so good for synchronization,
in contrast chemical communication, whose diffusion is spatially limited and
slow, is important in development. As for phylogenetic reasons, electrical
synapses may have used to be good for something and we have not figured how to
get rid of them. The fact is, that many mammalian neurons do form electrical
synapses.
At most sites, the function is to synchronize, i.e. the neurons are quasi reticular.
So, what would Cajal have said? " … [I]n accepting the most exaggerated
syncytial hypotheses … eveything that the physiologists, during 50 years of
dogged and fruitful investigation, have taught us concerning localization in
the nervous centers is left without an explanation. We would therefore precipitate
into chaos, into a discouraging nihilism …" ("…
aceptando
las hipótesis sinciciales más exageradas, y extendiéndolas a todo el sistema
nervioso, quedan sin explicación todos los reflejos musculares limitados, así
como las impresiones sensoriales concretas (cromáticas, acústicas, tactiles,
espaciales, etcétera), y en fin, todo cuanto durante los cincuenta años de
porfiada y fecunda investigación nos han enseñado los fisiólogos acerca de las
localizaciones en los centros nerviosos. Caeríamos, pues, en el caos, en un
nihilismo desalentador… ", as he wrote short before starting ¿Neuronismo o reticularismo?'s Conclusión).
But on the next
page, he adds: "I am neither exclusive nor dogmatic, I am proud of
retaining a mental flexibility which is not afraid of corrections. Neuronal
discontinuity … could sustain some exceptions." ("No somos
exclusivos ni dogmáticos. Tenemos a gala el conservar una flexibilidad mental
que no se avergüenza de rectificaciones. La discontinuidad neuronal,
evidentísima en innumerables ejemplos, pudiera padecer excepciones",
Cajal's words in ¿Neuronismo o
reticularismo?'s Conclusión). Gap junctions, indeed, connect cell cytoplasms on a
molecular scale, ~ 1 kDa or 1,5 nm to form a functional syncitium; the squid
giant axon and some septate axons are exceptions.
One may still mull over what Cajal said in the Nobel
Prize award ceremony: "Finally, the prize for Peace was awarded to the
American Theodore Roosevelt. This decision produced great surprise, specially
in Spain. It is not the acme of irony and humor to convert into a champion of
pacifism the man of the most impetuously pugnacious temperament and the most
determined imperialist that the United States have ever produced?" (p.
550). And, on Golgi (p. 553): "What a cruel irony of fate to pair, like
Siameses [sic] twins united by the shoulders, scientific adversaries of such
contrasting character!"

Further panoramas of the topic:
Santiago Ramón y
Cajal, Histology of the
Nervous System of Man and Vertebrates, N. Swanson, L.W. Swanson, trans.
(Oxford Univ. Press, New York, 1995).
Michael V.L.
Bennett and R. Suzanne Zukin, "Electrical Coupling and Neuronal
Synchronization in the Mammalian Brain," Neuron 41, 495–511, February 19, 2004.
Theodore H.
Bullock, Michael V. L. Bennett, Daniel Johnston, Robert Josephson, Eve Marder,
R. Douglas Fields, "The Neuron Doctrine, Redux," Science 310, 791-3 (2005).
_______
Copyright © 2006 by the
author. Este trabajo es un artículo de acceso público;
su copia exacta y redistribución por cualquier medio están permitidas bajo la
condición de conservar esta noticia y la referencia completa a su publicación
incluyendo la URL (ver arriba). / This is an Open Access article: verbatim copying
and redistribution of this article are permitted in all media for any purpose,
provided this notice is preserved along with the article's full citation and
URL (up front).

revista
Electroneurobiología
ISSN: 0328-0446